Carbon Capture &
Decarbonization
ANALYTICAL SOLUTIONS FOR CARBON CAPTURE & DECARBONIZATION
Cutting-Edge Gas Analyzers: solutions for measuring CO2
Welcome to our Carbon Capture and Decarbonization application page! Here, you will learn about the importance of carbon capture and decarbonization, and how our solutions can help you achieve your sustainability goals.
Our team of experts possesses extensive experience in carbon capture and decarbonization, and we are driven to assist our customers in realizing their sustainability targets. Whether you represent an industrial plant aiming to reduce your carbon footprint or a government agency seeking to implement effective carbon capture policies, our solutions and expertise are tailored to meet your unique requirements.
With a diverse range of products, technologies, and services, we help you identify the most effective solutions for your specific situation. Our solutions are designed to be scalable and cost-effective, and we prioritize close collaboration with our customers to ensure timely and budget-friendly project delivery.
If you are interested in learning more about our carbon capture and decarbonization solutions, please contact us today to schedule a consultation with one of our experts. Together, we can make a positive impact on the environment and help build a more sustainable future for generations to come.
- WHAT IS CARBON CAPTURE AND STORAGE (CCS)?
- WHAT ARE THE TYPES OF CARBON CAPTURE?
- HOW IS CO2 REMOVED IN CARBON CAPTURE?
- REDUCING GREENHOUSE GAS (GHG) EMISSIONS
- WHY IS CARBON CAPTURE AND STORAGE (CCS) ESSENTIAL?
- WHY DETECTING IMPURITIES IN CARBON DIOXIDE MATTERS?
- CARBON DIOXIDE APPLICATION IMPLICATIONS
- HYDROGEN PRODUCTION
-

WHAT IS CARBON CAPTURE AND STORAGE (CCS)?
Carbon Capture and Storage (CCS) is an essential component of reducing greenhouse gas emissions, especially in industries with high CO2 output, such as power generation, cement production, and steel manufacturing. By capturing and storing CO2, these industries can significantly reduce their carbon footprint and contribute to global efforts to limit climate change.
However, it is important to note that while carbon capture is a valuable tool in reducing CO2 emissions, it is not a silver bullet. It must be coupled with other measures, such as transitioning to renewable energy sources, improving energy efficiency, and promoting sustainable practices across various sectors, to achieve meaningful and lasting reductions in greenhouse gas emissions.
-

WHAT ARE THE TYPES OF CARBON CAPTURE?
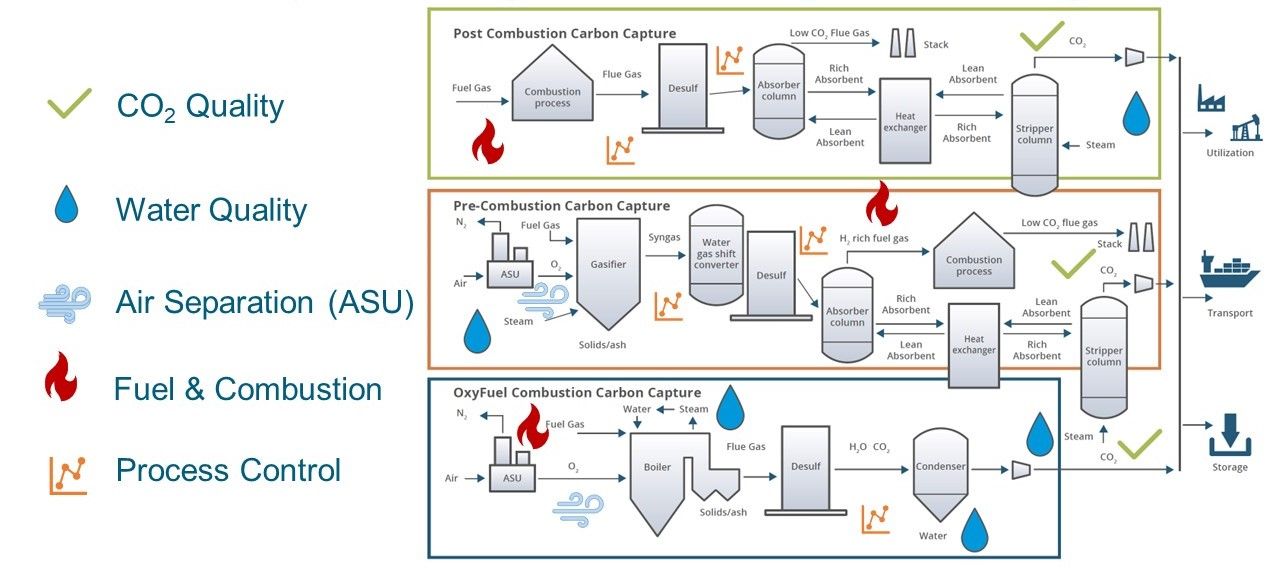
There are 3 Main “Types” of Carbon Capture.
- Post-Combustion Carbon Capture:
- How it works: Post-combustion carbon capture is a technology that captures carbon dioxide (CO2) emissions after the combustion process in power plants or industrial facilities. It is applied to the flue gases produced when burning fossil fuels.
- Capture Process: Flue gases are passed through a scrubbing system that contains a solvent or sorbent, which selectively captures the CO2. The captured CO2 is then separated from the solvent or sorbent, typically through a chemical reaction or temperature change.
- Applications: Post-combustion capture can be retrofitted onto existing power plants and industrial facilities, making it a valuable option for reducing emissions from existing infrastructure.
- Pre-Combustion Carbon Capture:
- How it works: Pre-combustion carbon capture is a technology that captures CO2 emissions before the combustion process occurs, typically in the context of gasification or hydrogen production.
- Capture Process: In pre-combustion capture, fossil fuels or other carbon-containing feedstocks are subjected to a gasification process. During this process, the carbon is converted into a synthetic gas (syngas), which contains CO2. The CO2 is separated from the syngas before combustion takes place.
- Applications: Pre-combustion capture is commonly used in integrated gasification combined cycle (IGCC) power plants and for hydrogen production. It is suitable for capturing CO2 from various feedstocks, including coal and biomass.
- Oxy-Fuel Combustion Carbon Capture:
- How it works: Oxy-fuel combustion carbon capture involves burning fossil fuels or other carbon-containing feedstocks in an environment enriched with oxygen (oxy-fuel combustion) to facilitate the capture of CO2 emissions.
- Capture Process: In oxy-fuel combustion, pure oxygen is used instead of air for combustion. This results in flue gases with a high concentration of CO2 and water vapor. The water vapor is condensed, leaving behind a concentrated CO2 stream, which can then be captured and stored.
- Applications: Oxy-fuel combustion carbon capture is often used in power generation and industrial processes. It offers high-purity CO2 capture and can be particularly suitable for industries with high-temperature processes.
Each of these carbon capture methods has its advantages and is suited to different applications and situations. The choice of the most appropriate method depends on factors such as the type of facility, the nature of the emissions source, and economic considerations. The ultimate goal is to reduce greenhouse gas emissions and combat climate change by capturing and storing CO2 emissions from various sources.
- Post-Combustion Carbon Capture:
-

HOW IS CO2 REMOVED IN CARBON CAPTURE?
Carbon dioxide (CO2) is removed in carbon capture processes through various mechanisms, depending on the specific method used. The primary goal is to capture and separate CO2 from the flue gases or gas streams generated by industrial processes. Here’s how CO2 is removed in carbon capture:
- Absorption:
- Post-Combustion Capture: In post-combustion carbon capture, the most common method is absorption. Flue gases from power plants or industrial facilities, which contain CO2 along with other gases like nitrogen and sulfur dioxide, are passed through a scrubbing system.
- Absorbent: The scrubbing system contains an absorbent solution, typically an amine-based solvent, that has a high affinity for CO2. As the flue gases pass through the solution, the CO2 molecules are chemically absorbed by the solvent, forming a carbon-rich solution.
- Chemical Reaction: The absorption process often involves a chemical reaction in which the CO2 reacts with the amine to form a stable compound. This compound is then separated from the solution in a subsequent step to release and capture the pure CO2.
- Adsorption:
- Pre-Combustion Capture: In pre-combustion carbon capture, adsorption is a common method. Here, carbon capture occurs before combustion, typically during gasification or hydrogen production.
- Adsorbent Material: Adsorbents, such as activated carbon or zeolites, are used to capture CO2 from the gas stream. These materials have a high surface area and can adsorb CO2 molecules through physical interactions.
- Regeneration: After adsorption, the adsorbent is typically regenerated by changing conditions, such as pressure or temperature. This releases the captured CO2 and allows the adsorbent to be reused for further capture cycles.
- Condensation:
- Oxy-Fuel Combustion Capture: In oxy-fuel combustion carbon capture, CO2 is captured by condensation. This method is used when fossil fuels are burned in an oxygen-enriched atmosphere.
- Water Vapor Condensation: The high concentration of CO2 in the oxy-fuel combustion flue gases is accompanied by a significant amount of water vapor. By cooling and condensing the water vapor, the CO2 is left in a concentrated form.
- Collection: The condensed water vapor is collected and separated from the CO2, leaving a stream of relatively pure CO2 for storage or utilization.
In all these methods, the captured CO2 is subsequently separated from the capture medium, purified, and then compressed for transportation and storage. The choice of carbon capture method depends on the specific application, the nature of the emissions source, and economic factors.
Once captured, the stored CO2 can be transported to suitable geological storage sites, such as depleted oil and gas reservoirs or saline aquifers, where it is permanently stored underground, or it can be used for various applications, including enhanced oil recovery or as a feedstock for industrial processes. Carbon capture is a crucial technology for mitigating greenhouse gas emissions and combating climate change.
- Absorption:
-

REDUCING GREENHOUSE GAS (GHG) EMISSIONS
Carbon capture and reducing greenhouse gas (GHG) emissions are integral components of the collective effort to combat climate change and create a sustainable future for several interconnected reasons:
- Mitigating Global Warming: GHGs, primarily carbon dioxide (CO2), methane (CH4), and nitrous oxide (N2O), are responsible for the greenhouse effect, trapping heat in the Earth’s atmosphere and leading to global warming. Reducing GHG emissions and capturing CO2 directly help slow down this process and limit the rise in global temperatures.
- Limiting Climate Change Impacts: Climate change caused by increased GHG emissions results in various adverse effects, including more frequent and severe weather events, rising sea levels, droughts, heatwaves, and disruptions to ecosystems. By reducing emissions and capturing CO2, we can mitigate these impacts and protect vulnerable communities and ecosystems.
- Preserving Biodiversity: Climate change threatens biodiversity by altering ecosystems and habitats, making it difficult for many species to survive. By curbing emissions, we can reduce the rate of habitat destruction and give species a better chance to adapt to changing conditions.
- Ensuring Food and Water Security: Climate change can affect agricultural productivity and the availability of freshwater resources, leading to food and water scarcity. Reducing GHG emissions can help stabilize these critical resources and ensure food and water security for growing populations.
- Protecting Public Health: Climate change contributes to air pollution, extreme heat events, and the spread of diseases, all of which pose risks to human health. Lowering GHG emissions can improve air quality and reduce health-related risks, benefiting public well-being.
- Economic Stability: Climate change can disrupt economies through damage to infrastructure, increased healthcare costs, and decreased agricultural productivity. Reducing GHG emissions helps mitigate these economic risks and fosters long-term stability and sustainable growth.
- Energy Transition: Shifting away from fossil fuels and embracing cleaner energy sources is essential for reducing emissions. Carbon capture can be an interim solution to reduce emissions from industries and power plants that are difficult to decarbonize rapidly. It complements renewable energy deployment and supports the transition to a low-carbon energy system.
- International Cooperation: Climate change is a global challenge that requires cooperation among nations. Reducing GHG emissions and implementing carbon capture technologies are essential components of international climate agreements such as the Paris Agreement, which aims to limit global warming to well below 2 degrees Celsius above pre-industrial levels.
- Long-Term Sustainability: Creating a sustainable future means considering the needs of future generations. By reducing GHG emissions and capturing carbon, we help ensure that Earth remains habitable and prosperous for generations to come.
- Innovation and Green Technologies: The pursuit of carbon capture and emission reduction technologies stimulates innovation and green technology development. These advancements can create new industries, generate jobs, and drive economic growth in a sustainable manner.
Carbon capture and reducing greenhouse gas emissions are integral components of the collective effort to combat climate change and create a sustainable future. Carbon capture technologies involve capturing carbon dioxide emissions from various sources, such as power plants and industrial facilities, before they are released into the atmosphere. These captured emissions can then be stored underground or repurposed for other industrial processes. Additionally, reducing greenhouse gas emissions involves implementing cleaner and more efficient energy practices, transitioning to renewable energy sources, and adopting eco-friendly technologies. By actively pursuing both carbon capture and emission reduction strategies, we can significantly decrease the amount of greenhouse gases in the atmosphere, mitigate global warming, and protect the planet’s delicate ecological balance for generations to come.
-

WHY IS CARBON CAPTURE AND STORAGE (CCS) ESSENTIAL?
Carbon Capture Storage (CCS) is a critical tool in the fight against climate change because it helps reduce the concentration of CO2 in the atmosphere. CO2 is a major greenhouse gas responsible for trapping heat and causing global warming. By capturing and storing CO2 emissions, CCS prevents them from contributing to the greenhouse effect and global temperature rise.
- Addressing Emissions from Hard-to-Decarbonize Sectors: In many sectors of the economy, such as heavy industry, aviation, and shipping, it is challenging to transition to entirely carbon-free energy sources in the near term. CCS allows these sectors to continue their operations while significantly reducing their carbon footprint.
- Supporting Energy Security: CCS can be applied to existing fossil fuel power plants and industrial facilities, extending the lifespan of these assets while making them more environmentally sustainable. This can help maintain energy security during the transition to cleaner energy sources.
- Balancing Renewable Energy Variability: Renewable energy sources like wind and solar are intermittent, making it necessary to balance their variability with reliable, dispatchable power sources. Natural gas power plants equipped with CCS can provide this reliability without the associated high carbon emissions.
- Ensuring Economic Viability: In regions heavily reliant on fossil fuels for their economy, CCS can help preserve jobs and economic stability during the transition to a low-carbon future.
- Technological Innovation: Continued research and development in CCS technology can lead to improved efficiency, cost reductions, and innovative solutions for capturing and utilizing CO2 emissions, such as carbon utilization in products or enhanced oil recovery.
Challenges and Considerations
While CCS is a promising technology, it is not without challenges and considerations, including cost, infrastructure requirements, and public acceptance. Additionally, the long-term effectiveness and safety of CO2 storage must be carefully managed to avoid leakage and ensure the integrity of storage sites.
Carbon Capture and Storage is an essential tool in reducing greenhouse gas emissions because it allows us to capture and store CO2 emissions from hard-to-decarbonize sectors, mitigating the effects of climate change, and supporting the transition to a more sustainable and low-carbon future. While it is not a silver bullet, CCS is a valuable component of a comprehensive strategy to combat climate change.
-

WHY DETECTING IMPURITIES IN CARBON DIOXIDE MATTERS?
Selecting the best-suited gas analyzers for detecting impurities in carbon dioxide (CO2) streams within Carbon Capture and Storage (CCS) applications is crucial to ensure the accuracy of measurements and maintain the safety and efficiency of CCS processes. Here are some key reasons why specific gas analyzers are well-suited for this purpose:
- Precision and Sensitivity: Gas analyzers designed for CCS applications are often highly precise and sensitive, capable of detecting impurities at low concentrations. This is essential because even trace amounts of certain impurities, such as hydrogen sulfide (H2S), can be harmful or corrosive.
- Real-Time Monitoring: Many CCS operations require real-time monitoring of impurity levels to respond quickly to any deviations from acceptable limits. Gas analyzers equipped with continuous monitoring capabilities allow for immediate detection and response to impurity spikes.
- Multi-Gas Capability: CCS streams can contain a variety of impurities, including nitrogen (N2), oxygen (O2), sulfur compounds, and water vapor. Multi-gas analyzers can simultaneously detect and quantify multiple impurities, providing comprehensive information about the composition of the CO2 stream.
- Low Detection Limits: Gas analyzers with low detection limits are essential for accurately quantifying impurities, especially when stringent regulatory requirements or safety considerations demand precise measurements.
- Durability and Reliability: CCS operations often take place in harsh environments, such as pipelines and storage facilities. Gas analyzers designed for CCS applications should be robust, durable, and reliable to withstand challenging conditions.
- Response Time: Rapid response time is crucial for detecting impurity fluctuations quickly. Analyzers with fast response times can help prevent safety incidents and maintain the quality of captured CO2.
- Calibration and Accuracy: Gas analyzers used in CCS must be regularly calibrated to ensure accuracy. Some analyzers offer features for easy and frequent calibration to maintain measurement precision.
- Remote Monitoring and Control: In some cases, CCS facilities may require remote monitoring and control of gas analyzers. Analyzers with remote connectivity options enable operators to monitor and adjust settings from a central location, enhancing operational efficiency.
- Compliance with Standards: Gas analyzers used in CCS should comply with relevant industry standards and regulations. Ensuring that analyzers meet these standards is essential for regulatory compliance and obtaining necessary permits.
- Data Logging and Reporting: Many CCS projects require extensive data logging and reporting capabilities. Gas analyzers equipped with these features can help maintain detailed records of impurity levels for analysis and reporting purposes.
-

CARBON DIOXIDE APPLICATION IMPLICATIONS
The presence of impurities in carbon dioxide (CO2) streams within Carbon Capture and Storage (CCS) processes is a critical consideration due to its various implications for the overall effectiveness and safety of CCS schemes.
- Types of Impurities:Impurities in CO2 streams can come in various forms, including but not limited to nitrogen (N2), sulfur compounds (such as hydrogen sulfide, H2S), oxygen (O2), water vapor (H2O), and trace elements like heavy metals. The specific types and concentrations of impurities depend on the source of CO2, such as power plants, industrial processes, or natural gas reservoirs.
- Effects on Transportation:Impurities can affect the physical properties of CO2, altering its behavior during transportation. For instance, the presence of water vapor can lead to the formation of carbonic acid, which can be corrosive to pipelines and storage infrastructure.
- Safety Considerations:Impurities like H2S and O2 can pose significant safety risks. H2S is toxic, and even small concentrations can be lethal, while O2 can increase the risk of combustion or explosion.
- Corrosion Control:Impurities can accelerate corrosion in pipelines and storage facilities. Effective corrosion control measures must be implemented to ensure the integrity and longevity of the infrastructure.
- Environmental Impact:The release of impurities during transportation or storage incidents can have environmental consequences. For instance, the release of H2S can be harmful to the environment and nearby communities.
- Regulatory Compliance:Many regions have strict regulations regarding the permissible levels of impurities in CO2 streams for CCS. Ensuring compliance with these regulations is essential for obtaining permits and maintaining the social license to operate.
- Purity Requirements:The purity of CO2 is critical for various industrial applications. In some cases, such as food and beverage manufacturing or enhanced oil recovery, high-purity CO2 is required. Impurities must be removed or reduced to meet these standards.
- Cost Considerations:The removal of impurities can be energy-intensive and costly. The choice of impurity removal methods and their efficiency can impact the overall economics of CCS projects.
- Monitoring and Analysis: Regular monitoring and analysis of CO2 streams are essential to assess the levels of impurities and ensure compliance with quality and safety standards. This may involve the use of sensors, analytical equipment, and laboratory testing.
-

HYDROGEN PRODUCTION
Hydrogen and carbon capture and storage (CCS) represent a potent combination in the global efforts to combat climate change. Hydrogen, often referred to as blue hydrogen when produced from natural gas with CCS, offers a low-carbon energy carrier that can replace fossil fuels in sectors like industry and transportation. When used in conjunction with CCS technology, the carbon emissions generated during hydrogen production can be captured, transported, and stored underground in geological formations, preventing them from entering the atmosphere. This synergistic approach, known as blue hydrogen with CCS, not only produces low-carbon hydrogen but also reduces overall greenhouse gas emissions by capturing and securely storing carbon dioxide (CO2). By integrating hydrogen and CCS, we have the potential to significantly mitigate carbon emissions while enabling the large-scale deployment of hydrogen as a clean energy solution in a wide range of applications.
Carbon Capture & Sequestration Analyzer Technologies
Quality Analyzers

Compression, transportation, storage and utilization are all heavily impacted by impurities in the CO2. Water must always be removed; combustion components and solvent breakthrough is an issue, and air leaks pose potential problems at every stage downstream. This makes real-time, automated CO2 purity monitoring essential to protect process equipment and compressors, to ensure the gas meets pipeline specifications, and to enable accurate reporting for tax credits. Process Insights offers ideal solutions for measuring CO2, along with the required impurities. Our application experts work with your team to select the right set of technologies for your carbon capture project.
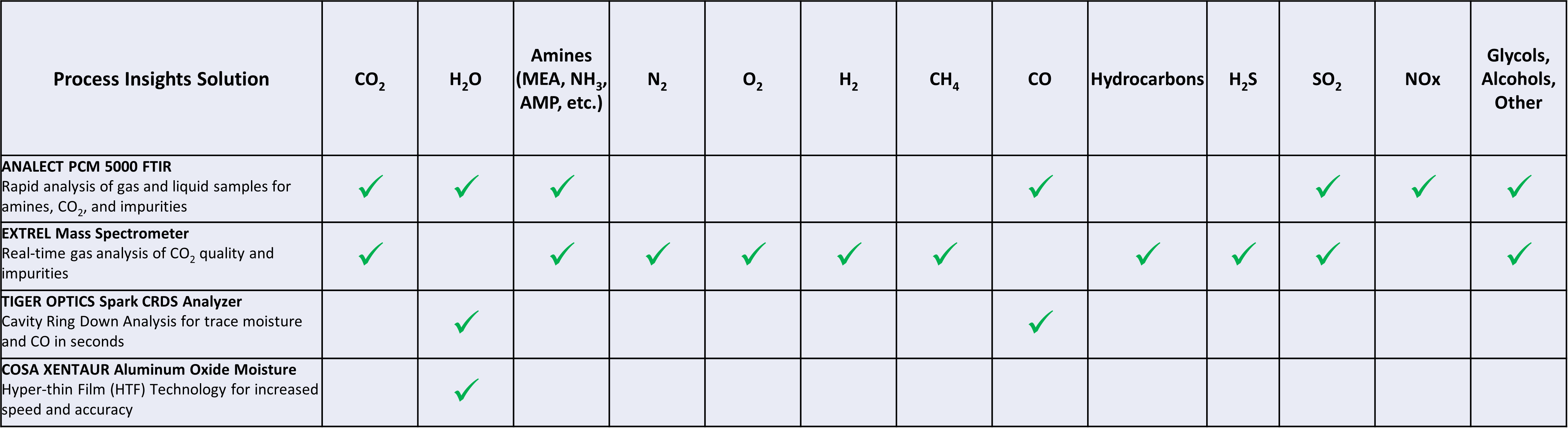
ANALECT® PCM 5000™
FTIR Analyzer for Physical, Chemical & Compositional Properties of Liquids, Solids & Gases
EXTREL™ MAX300-RTG™ 2.0
Industrial Process Quadrupole Mass Spectrometer for Continuous, Online Gas Stream Monitoring
TIGER OPTICS™ SPARK™
PPB-Level Trace Moisture Detection Cavity Ring-Down Spectroscopy (CRDS) Gas Analyzer
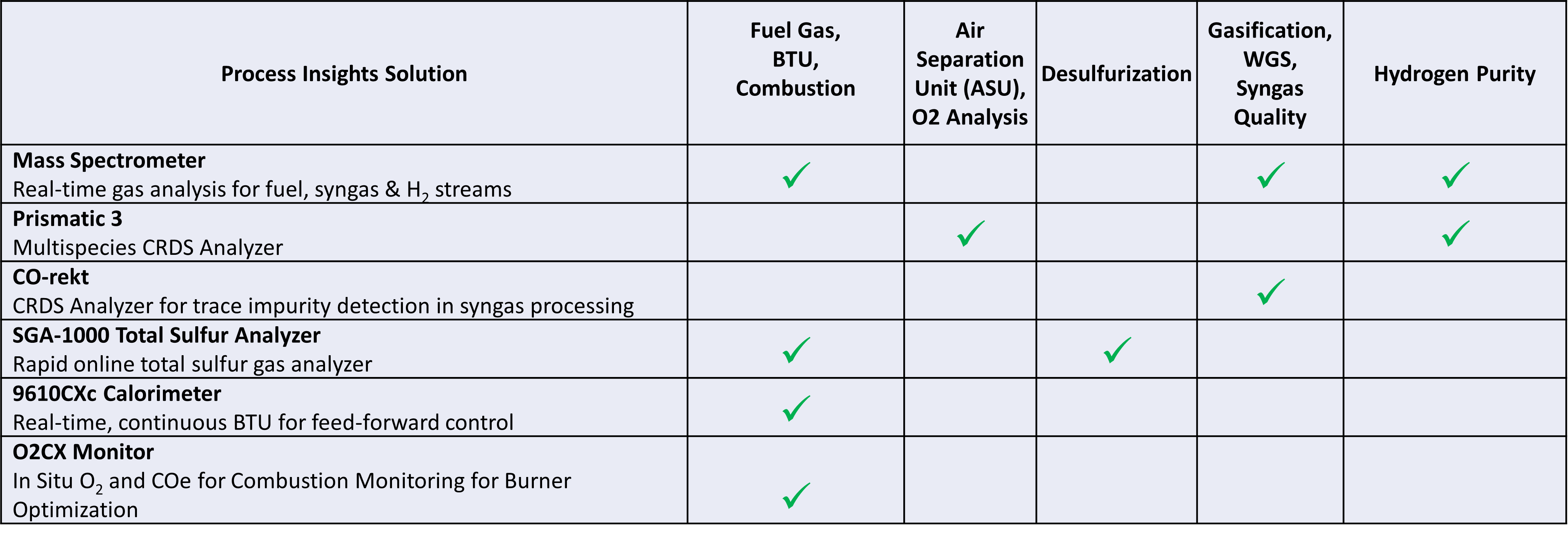
ASU, Combustion & Process Control

Real-time analyzers for the gases used in energy and syngas production and carbon capture enable confident, effective process control and maximum operational efficiency. This includes O2 purity for Air Separation Units (ASU), gasification and syngas quality monitoring, as well as feed-forward control for industrial combustion and process reactors. At Process Insights we match analyzer solutions from a wide range of technologies to the specific set of critical sample streams upstream and downstream of the carbon capture process.
Water Analysis Applications

ANALYSIS
- Fast, online water quality analysis
- Water influent and effluent discharge control
- Process control, high purity, oil in water, cooling water, condensate return, water-steam cycles, and boiler feed water and environmental compliance
- Continuous, online water analysis for total organic carbon (TOC), chemical oxygen demand (COD) and biological oxygen demand (BOD)
- Online determination of TOC (TRUE TOC), TC, TIC, NPOC, DOC, POC/VOC
APPLICATIONS
- Harsh wastewater
- Pure and process water
- Ultra-pure water
- Industrial wastewater
- Environmental compliance
- Chemicals
- Refinery
- Manufacturing
- Food and beverage
LAR™ QuickTOCultra™
Water Analyzesrs for Harsh Wastewater Applications Measuring Total Organic Carbon (TOC)














