Online NIR Water Measurements in Liquid Samples
Online NIR Water Measurements in Liquid Samples
Water is perhaps the most common measurement made in the near infrared (NIR). This is due to its strong effect on product properties and chemical reactivity of the starting materials. From an analytical perspective, water is easy to analyze due to its relatively strong signal compared to the hydrocarbon background.
Moreover, because water is commonly analyzed with a single wavelength, photometers are the instrument of choice. The purpose of this application note is to show you how we arrive at recommending a system, that is, a photometer with the proper wavelengths and a fiber optic probe with an appropriate sample path length.
- (1) background hydrocarbon spectral characteristics, (2) concentration range of water and desired analytical precision, (3) potential interference from hydroxyl, (4) sample temperature variations, and (5) sample clarity. These considerations affect the choice (and price) of the appropriate photometer and probe system.
- (1) provide maximum sensitivity, (2) select wavelength(s) to keep the absorbance below 1.2 Absorbance Units (AU), (3) minimize interference due to background hydrocarbon variations and sample temperature changes, and (4) use an optical path of >1 mm in the fiber optic probe for ease of cleaning and minimal entrapment of bubbles and particles.
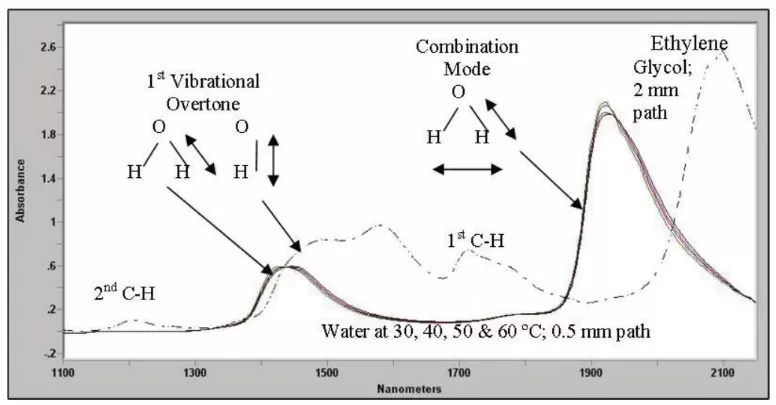
Figure 1 Location of various NIR absorbance peaks for water relative to the absorbance of Ethylene Glycol.
Rule-of-Thumb
The 1900 nm region is generally used for <1% water. With our ClearView® db photometer, a precision near ±20 ppm can be achieved. The 1400 nm region is used for >1% water and a precision of near ±100 ppm is attainable. For process analyzers, long-term (monthly) photometric drift is the most meaningful measure of “precision”. With a ClearVIew db photometer, this is typically <500μAU.

SALES | TRAINING | SERVICE
Americas +1.916.638.4944
EMEA +49 69 20436910
China/APAC +86 400 086 0106
USEFUL NIR UV-VIS Troubleshooting Guides & Technical Resources
OH vs. Water
The 1400 nm region contains the 1st overtone of the O-H stretch in water and hydroxyl, as shown above for water and ethylene glycol. Mixtures of water and alcohols, therefore, have over lapping features due to water and hydroxyl in this region. Water additionally has a unique peak near 1900 nm due to a combination of its O-H stretch and its H-O-H bending. The large peak near 2100 nm is a combination of O-H and C-H in the glycol. Notice that this pure glycol lacks an appreciable peak near 1900 nm where molecular water absorbs. With equivalent optical paths, you can see how much stronger water is compared to the C-H features.
Precision
The water peak at 1900 nm is about five times as large as its peak at 1430 nm. For example, 1 mAU in the 1900 nm region for n-propanol corresponds to about 20 ppm water for a 1 cm optical path, and about 90 ppm near 1430 nm. For water in methylisobutyl ketone (MIBK) or tetrahydrofuran (THF) these values are about 12 and 85 ppm/mAU, respectively. By doubling the optical path to 2 cm, if possible, the precision is also doubled. Take a conservative 3s drift of 1.5 mAU for a ClearView db, and we arrive at ±20 ppm at 1900 nm and ±125 ppm at 1430 nm with a 1 cm pathlength in situ probe.
The absorbance from an organic liquid near 1900 nm is highly variable. CCl4 has no appreciable absorbance in this region. Optical paths from 10 to 20 cm can be used to achieve <1 ppm precision. In contrast, many alcohols have significant absorbance. For example, ethylene glycol at 1900 nm has about 1.4 AU for a 1 cm optical path. Therefore, we could use a 5 mm optical path in a fiber optic probe to provide a 0.7 AU “baseline” absorbance in ethylene glycol. At 5 mm, the precision for water in ethylene glycol is about 24 ppm/mAU, so 1% water would add about 0.4 AU to the 0.7 AU resulting in an absorbance near 1.1 AU. You can see that adding >1% water to ethylene glycol, even in a 5 mm probe, would lead to an absorbance larger than our 1.2 AU desired limit. Thus, 2% water in ethylene glycol is measured with a shorter path, such as 2 or 3 mm, with some sacrifice in precision to achieve the greater concentration range.
Sample Clarity
A photometer computes the absorbance between the water wavelength and a “reference” wavelength (= -log Water/Reference) and converts that into water concentration. The “reference” wavelength is typically chosen in a “valley” which has little absorbance change, such as 1300 nm. The presence of light scattering entities entrained in the sample stream, such as bubbles, particles or immiscible phases, can “tilt” the entire spectrum between the water and “reference” wavelengths. Since we are already quantifying mAU changes at the water peak, it does not take much of a “tilt” to significantly impair a water calibration. The greater the wavelength difference, the more the effect, such as at 1900 nm. We can always choose a “reference” wavelength on either side of the 1900 nm water to minimize this effect, but it is still important to install particle filters and sample temperature control.
Temperature Effects
The top of the water peak near 1430 nm is shown in Figure 2. These spectra represent water at different temperatures from 30 to 60 oC. They intersect at 1430 nm. There is an analogous intersection on the 1900 nm peak shown on the previous page. This means that different temperatures have different calibration curves. In some of our studies of percent water in acids in the 1400 nm region, a 1 oC change can lead to a 0.1% (1000 ppm) change in the calculated water concentration. There are several methods to minimize sample temperature effects. The piping in a side stream can be heat traced or the wavelength at the spectral intersection point can be used for the calibration. Perhaps the most common solution is to measure the sample temperature with an RTD sensor or thermocouple near the probe and send the signal back to the ClearView® db photometer. The software is equipped to correct water calibrations for sample temperature variations.
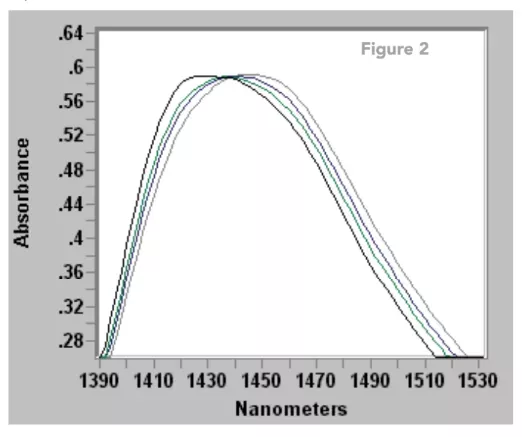
Figure 2
By considering the issues raised above, we can provide you with a cost-effective solution to obtain the desired sensitivity for water over the concentration range of interest in most organic liquids. Please contact us for questions or inquiries.
Our comprehensive GUIDED WAVE NIR UV-VIS process and lab analyzer offer optically matched components and a meticulously planned calibration approach, ensuring long-term efficiency and cost savings. Our advanced systems are engineered for continuous online operation, delivering real-time data of laboratory-grade quality, even in the harshest processing plant conditions.

