COD Water Analysis
Chemical Oxygen Demand
What is Chemical Oxygen Demand (COD)?
We are the TOC Leader™ | Monitor TOC, COD, BOD, TN, and Toxicity Impurities in all Types of Water
Chemical Oxygen Demand (COD) is defined as the amount of oxygen equivalents consumed in the chemical oxidation of organic matter by strong oxidant (e.g., potassium dichromate). The COD value indicates the amount of oxygen which is needed for the oxidation of all organic substances in water in mg/l or g/m3. Chemical oxygen demand (COD) is an indirect measurement of the amount of organic matter in a sample. With testing COD, you can measure virtually all organic compounds that can be digested by a digestion reagent.
COD water analysis is critical in wastewater for determining the amount of waste (contamination) in the water. Waste that’s high in organic matter requires treatment to reduce the amount of organic waste before discharging into receiving waters. Why does this matter? If wastewater treatment facilities do not reduce organic content of the wastewater before it reaches natural waters, microbes in the receiving water will consume the organic matter. As a result, microbes will consume the oxygen in the receiving water to breakdown the organic waste. This oxygen depletion is called eutrophication and can lead to the death of animal life.
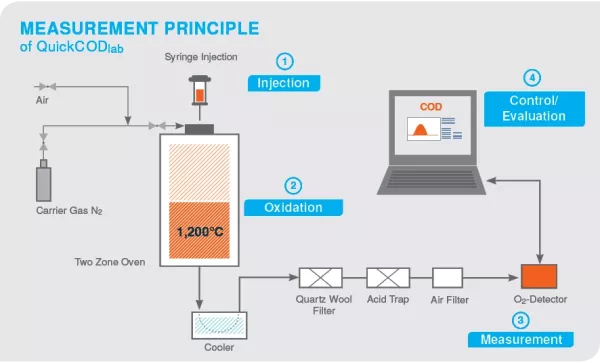
Chemical oxygen demand tests are typically performed on wastewater. The pollution level is calculated by measuring the amount of organic matter in the water. Water with too much organic material can have a negative effect on the environment in which the wastewater is discharged. The COD (Chemical Oxygen Demand) is closely related to the laboratory standard method named Dichromate-Method. With this method the chemical oxygen demand is determined during chromic acid digestion of organic loads in wastewater. Based on this method the COD became a commonly used sum parameter in wastewater analysis. It is used for planning of wastewater treatment plants, for controlling the cleaning efficiency and for the calculation of wastewater taxes.
Monitoring total organic carbon (TOC), biological oxygen demand (BOD), and total nitrogen (TN) is essential for maintaining water quality and ensuring compliance with environmental regulations.
OTHER WATER APPLICATIONS
For a complete range of analytical instrumentation, applications, systems, and service options, we will work to match your needs and budget and provide the optimal, and most stable process analysis solution for your application.
SALES | TRAINING INQUIRIES
AMERICAS: info@process-insights.com
EMEAI: info.emeai@process-insights.com
APAC: info.apac@process-insights.com
CHINA: info.cn@process-insights.com
SERVICE | TECHNICAL INQUIRIES
-

WHAT IS chemical oxygen demand?
Chemical Oxygen Demand (COD) is a measure of the amount of oxygen required to chemically oxidize organic and inorganic compounds present in water. It provides an estimation of the quantity of pollutants that can be oxidized by strong chemical oxidants.
COD is determined by oxidizing a water sample with a strong oxidizing agent, such as potassium dichromate (K2Cr2O7), in the presence of a strong acid. During the oxidation process, the organic and inorganic compounds present in the water are chemically broken down, converting them into carbon dioxide (CO2) and water (H2O). The amount of oxidizing agent consumed is then measured, which reflects the COD of the water sample.
The measurement of COD in water serves several important purposes:
- Water Quality Assessment: COD is a widely used parameter for assessing the overall pollution level and the organic content of water. High COD values indicate the presence of significant amounts of organic pollutants, such as wastewater, industrial effluents, or organic chemicals. Monitoring COD helps in evaluating the water quality and identifying sources of pollution.
- Wastewater Treatment Efficiency: COD measurements are essential for assessing the performance and efficiency of wastewater treatment processes. By measuring the COD of influent (raw) and effluent (treated) wastewater, treatment plant operators can evaluate the effectiveness of the treatment process in removing organic pollutants. Monitoring COD aids in optimizing treatment operations and ensuring compliance with regulatory standards.
- Regulatory Compliance: Many countries and regulatory bodies have established COD limits and guidelines for discharges into water bodies. Monitoring COD helps ensure compliance with these regulations, which are designed to protect water quality and prevent excessive pollution.
- Process Control and Optimization: COD measurements provide valuable information for process control and optimization in various industries. By monitoring COD in industrial wastewater, companies can identify and mitigate potential pollution sources, adjust treatment processes, and minimize environmental impacts.
- Research and Environmental Studies: COD data is utilized in scientific research, environmental monitoring, and studies on water pollution. It helps in understanding the impact of organic pollutants on water ecosystems, tracking pollution trends, and developing strategies for pollution prevention and remediation.
-

what is the CORRELATION BETWEEN TOC & COD?
Total Organic Carbon (TOC) and Chemical Oxygen Demand (COD) are both parameters used in water analysis to assess the organic content and pollution level of water. While there is a correlation between TOC and COD, it is important to understand their differences and the relationship between them.
TOC measures the total amount of carbon in a water sample, including both organic and inorganic carbon compounds. It quantifies the concentration of carbon present in the sample, regardless of its oxidizability. TOC analysis involves the measurement of the carbon content directly, typically using high-temperature combustion or wet chemical oxidation methods.
COD, on the other hand, measures the amount of oxygen required to chemically oxidize both organic and inorganic compounds in a water sample. It provides an estimation of the oxidizable organic content of the sample. COD analysis involves the use of a strong oxidizing agent to oxidize the organic compounds in the sample, and the consumed oxidizing agent is then measured to determine the COD value.
While both TOC and COD provide information about the organic content of water, they differ in terms of their measurement principles and the scope of compounds they quantify. TOC measures the total carbon content, including both organic and inorganic carbon, while COD specifically focuses on the oxidizable organic content.
In many cases, there is a positive correlation between TOC and COD. This means that as the TOC concentration increases, the COD value also tends to increase. This correlation occurs because the majority of organic compounds contribute to both the TOC and COD values. However, it is important to note that the correlation may not be perfect due to the presence of inorganic carbon compounds, non-oxidizable organic compounds, and variations in the composition of organic pollutants.
-

WHY SHOULD YOU MONITOR COD IN WATER?
Monitoring Chemical Oxygen Demand (COD) in water is important for several reasons:
- Water Quality Assessment: COD measurement is a valuable tool for assessing the overall pollution level and organic content of water. High COD values indicate the presence of significant amounts of organic pollutants, such as wastewater, industrial effluents, or organic chemicals. Monitoring COD helps in evaluating the water quality and identifying sources of pollution.
- Wastewater Treatment Efficiency: COD measurements are essential for evaluating the performance and efficiency of wastewater treatment processes. By measuring the COD of influent (raw) and effluent (treated) wastewater, treatment plant operators can assess the effectiveness of the treatment process in removing organic pollutants. Monitoring COD aids in optimizing treatment operations and ensuring compliance with regulatory standards.
- Regulatory Compliance: Many countries and regulatory bodies have established COD limits and guidelines for discharges into water bodies. Monitoring COD helps ensure compliance with these regulations, which are designed to protect water quality and prevent excessive pollution. By monitoring COD levels, industries and wastewater treatment facilities can take corrective measures to meet regulatory requirements.
- Pollution Source Identification: Monitoring COD in water can help identify specific sources of pollution. By analyzing the COD levels in different areas or water bodies, it becomes possible to pinpoint the locations or industries contributing to the elevated COD values. This information is crucial for implementing targeted pollution control measures and holding responsible parties accountable.
- Environmental Impact Assessment: High levels of organic pollutants, as indicated by COD measurements, can have adverse effects on aquatic ecosystems. The decomposition of organic matter consumes oxygen in water, leading to oxygen depletion and potentially harming aquatic life. By monitoring COD, environmental scientists and regulators can assess the potential impact of organic pollution on the health of aquatic ecosystems and take necessary mitigation measures.
- Process Optimization: Monitoring COD provides valuable data for process optimization in various industries. By tracking COD levels in wastewater generated by industrial processes, companies can identify areas of excessive organic waste generation and implement measures to minimize pollution. This helps improve the overall environmental performance of industries and reduce their impact on water resources.
-

THERMAL OXIDATION (HIGH TEMPERATURE METHOD)
The thermal oxidation method, also known as the high-temperature method, is a technique used in water analysis to determine various parameters, such as total organic carbon (TOC) or total carbon (TC). It involves the oxidation of organic and inorganic carbon compounds present in a water sample at high temperatures.
In this method, a water sample is introduced into a combustion furnace or reactor, where it is heated to temperatures typically ranging from 850°C to 1,200°C in the presence of an oxidizing agent, such as oxygen or ozone. The high temperatures and oxidizing conditions cause the carbon compounds in the sample to oxidize completely, converting them into carbon dioxide (CO2).
Our LAR high temperature thermal oxidation method offers several advantages in water analysis:
- Versatility: It can be used for the determination of various carbon-related parameters, including total organic carbon (TOC), total inorganic carbon (TIC), and total carbon (TC).
- Wide Measurement Range: The high-temperature method can measure carbon concentrations over a wide range, from low levels (parts per billion) to high levels (percentage levels).
- Sample Matrix Compatibility: The thermal oxidation method is applicable to a broad range of water sample matrices, including drinking water, wastewater, surface water, and industrial process water.
- Complete Carbon Oxidation: The high temperatures used in the combustion process ensure the complete oxidation of carbon compounds, providing accurate and reliable measurements.
-

ELECTROCHEMICAL MEASUREMENT PRINCIPLE
The electrochemical water measurement principle is a technique used to analyze and measure various parameters in water based on electrical signals generated during specific electrochemical reactions. It involves the use of electrodes and the measurement of electrical potential, current, or impedance to determine the concentration or properties of certain compounds or ions in water.
The principle is based on the fact that certain chemical reactions or interactions produce changes in electrical properties, which can be quantified and correlated to the analyte concentration. Here are some key aspects of the electrochemical water measurement principle:
- Electrodes: Electrodes are essential components of electrochemical measurements. They are typically made of conductive materials, such as metals or metal oxides, and are designed to interact with the analyte of interest. Common types of electrodes used in water analysis include pH electrodes, ion-selective electrodes (ISEs), and conductivity electrodes.
- Redox Reactions: Electrochemical measurements often involve redox (reduction-oxidation) reactions, where the analyte undergoes a chemical change involving the transfer of electrons. These reactions occur at the surface of the electrodes and result in the generation or consumption of electrical current or potential.
- Ion-Selective Electrodes (ISEs): Ion-selective electrodes are specifically designed to selectively interact with a particular ion in the water sample. They contain an ion-specific membrane that allows the passage of only the target ion while blocking other ions. The change in potential or current generated by the ion-selective electrode is proportional to the concentration of the target ion in the water.
- pH Measurement: pH measurement is an important application of the electrochemical principle. pH electrodes, commonly known as glass electrodes, are sensitive to changes in hydrogen ion concentration in water. The potential difference between the reference electrode and the pH electrode is used to determine the pH value of the water sample.
- Conductivity Measurement: Conductivity measurement is based on the ability of water to conduct electrical current due to the presence of ions. Conductivity electrodes measure the electrical conductivity of water, which is related to the concentration of ions present. The higher the ion concentration, the higher the conductivity.
- Amperometry and Voltammetry: Amperometry and voltammetry are electrochemical techniques that measure the current flowing between electrodes as a result of a redox reaction. These methods are used to quantify specific analytes in water by applying a fixed potential or scanning the potential over a range of values.
OUR WATER QUALITY ANALYZERS BY INDUSTRY

AEROSPACE & GOVERNMENT

LAB & RESEARCH










