Thermogravimetric Analysis/Mass Spectrometry (TGA-MS)

Thermogravimetric Analysis/Mass Spectrometry (TGA-MS)
An Extrel MAX300-EGA was coupled with a NETZSCH TG 209 F1 Libra to Perform Evolved Gas Analysis
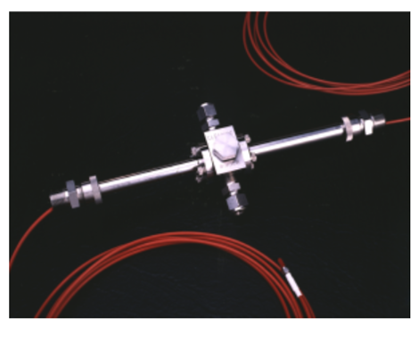
The heated transfer line of the MAX300-EGA™, a quadrupole mass spectrometer designed for evolved gas analysis, was connected to the off-gas port of a NETZSCH® TG 209 F1 Libra® thermobalance. A variety of samples were analyzed and the combination of the two technologies allowed for simultaneous thermal characterization and quantitative analysis of the compounds in the furnace exhaust.
Thermogravimetric analysis (TGA) is a powerful technique that has been used for many years to characterize solid and liquid samples. The mass of the sample material is monitored while it is heated. By using a high precision balance and carefully controlling the heating process, researchers are able to plot mass loss as a function of temperature. TGA is widely used in the study of polymers, pharmaceuticals and petrochemicals to determine degradation temperatures, characterize thermal decomposition, and monitor solvent and moisture content.
Additional information about sample composition and thermal behavior can be obtained by analyzing the gases that leave the material as it is heated. This allows the researcher to determine not only the temperature at which a mass loss occurs, but also the molecular structures involved. Evolved Gas Analysis (EGA) is commonly carried out via a variety of analytical techniques, but in all cases the integrity of the gas stream must be protected. It must be kept hot and moved quickly to the gas analyzer to prevent condensation and chemical interactions.
The NETZSCH TG 209 F1 Libra is a vacuum tight TGA, making it ideal for connecting to a mass spectrometer. The Libra is equipped with an automatic sample changer and can reach temperatures up to 1100°C. It measures sample mass to a resolution of 0.1 μg. The Libra’s heated adapter was connected to the transfer line of the Extrel MAX300-EGA. The interface is differentially pumped for rapid clearing and heated to 200°C to prevent condensation; it provides a low volume, chemically inert sample path from the TGA all the way into the mass spectrometer’s ionizer.
The MAX300-EGA is a quadrupole mass spectrometer optimized for evolved gas analysis in a laboratory setting. It is capable of scanning from 1-500 amu and features the Extrel 19 mm mass filter for high analytical repeatability and long-term stability. The Questor5 software allows the system to perform qualitative analysis for sample characterization, or quantitative analysis, measuring concentrations from 100% down to 10 ppb. In addition to the transfer line, a MAX300-EGA is equipped to import a start-of-heating signal from the TGA and can be configured to perform calculations and trend data or output the data for viewing and manipulation on a different platform.
Polystyrene Decomposition: Detection of High-Mass Fragments
The furnace of the Libra was loaded with 0.94 mg of polystyrene and heated to over 600°C. The breakdown of the sample was monitored to determine the MAX300’s sensitivity to the small signals generated by high-mass hydrocarbons in the off-gas. Although the TGA records the decomposition of the polystyrene as a single weight loss beginning at 290°C, the MAX300 is able to show that the evolution of several compounds has occurred.
It is generally difficult to keep larger molecules from dropping out of an evolved sample once it has left the furnace, but the mass spectrum at 39.75 minutes clearly shows the presence of styrene in the off gas (Fig. 3. B), as well as the much smaller signal generated by methyl styrene.
The mass of each component in the gas was calculated for comparison to data from the TGA’s balance. Even the relatively small, 60 μg, loss that occurred as moisture left the sample was easily measured and quantified by the mass spectrometer. The MAX300 was also able to individually determine the amount of carbon monoxide and carbon dioxide that, combined, resulted in the second mass loss. While the thermal breakdown of calcium oxalate is well documented, the ability of the MAX300 to perform similar quantitative separations can be used to better understand a complex decomposition featuring the simultaneous evolution of multiple unknown compounds.
Further Applications for the MAX300-EGA
The data gathered from the effluent of the TGA 209 F1 Libra indicates that the MAX300-EGA is a powerful tool for evolved gas analysis. The sensitivity, resolution and quantitation demonstrated during the tests indicate the instrument’s potential for other evolved gas applications. In its standard configuration or equipped with the 300 or 400°C transfer line upgrades, the MAX300-EGA could be used to quantify solvent loss in a pharmaceutical sample, detect trace VOCs, or monitor the gas exiting a microreactor.